Jan 15, 2024Determine the correct chemical formulas for each reactant and product. Write the skeleton equation. Count the number of atoms of each element that appears as a reactant and as a product. If a polyatomic ion is unchanged on both sides of the equation, count it as a unit.
What is the end product of a glycolysis reaction? – Quora
Label each reactant and product (as “reactant” or “product“) in the given chemical reaction. CH4 + 2 O2 CO2+ 2 H2O Question 2 of 25

Source Image: ask.learncbse.in
Download Image
Label each reactant and product in the given chemical reaction. Chemical reaction: A chemical reaction is a process in which two or more elements or compounds (the reactants)

Source Image: studypool.com
Download Image
Converting Mass of Reactant to Mass of Product — Examples – Expii Label each reactant and product in the given chemical reaction. Click the card to flip 👆 A reactant is an element or compound participating in a chemical reaction that undergoes a chemical change, and is shown to the left of the reaction arrow.

Source Image: studypool.com
Download Image
Label Each Reactant And Product In The Given Chemical Reaction.
Label each reactant and product in the given chemical reaction. Click the card to flip 👆 A reactant is an element or compound participating in a chemical reaction that undergoes a chemical change, and is shown to the left of the reaction arrow. All chemical reactions—including a candle burning—involve reactants and products. Reactants are substances that start a chemical reaction. Products are substances that are produced in the reaction. When a candle burns, the reactants are fuel (the candlewick and wax) and oxygen (in the air). The products are carbon dioxide gas and water vapor.
SOLUTION: Chemical reaction prashant kirad – Studypool
3 years ago Each oxygen atom fulfills its octet by bonding with another. Oxygen is part of the special group of elements whose atom’s bond to each other called elemental diatomic molecules. The most common examples being H2, N2, O2, F2, and Cl2. Whenever you see oxygen or oxygen gas in a problem, you can assume they mean O2. Reactants and chemical reaction products. | Download Scientific Diagram
Source Image: researchgate.net
Download Image
CollisionTheorySE Revised For MYP-Gizmos | PDF | Reaction Rate | Chemical Reactions 3 years ago Each oxygen atom fulfills its octet by bonding with another. Oxygen is part of the special group of elements whose atom’s bond to each other called elemental diatomic molecules. The most common examples being H2, N2, O2, F2, and Cl2. Whenever you see oxygen or oxygen gas in a problem, you can assume they mean O2.

Source Image: scribd.com
Download Image
What is the end product of a glycolysis reaction? – Quora Jan 15, 2024Determine the correct chemical formulas for each reactant and product. Write the skeleton equation. Count the number of atoms of each element that appears as a reactant and as a product. If a polyatomic ion is unchanged on both sides of the equation, count it as a unit.
Source Image: quora.com
Download Image
Converting Mass of Reactant to Mass of Product — Examples – Expii Label each reactant and product in the given chemical reaction. Chemical reaction: A chemical reaction is a process in which two or more elements or compounds (the reactants)

Source Image: expii.com
Download Image
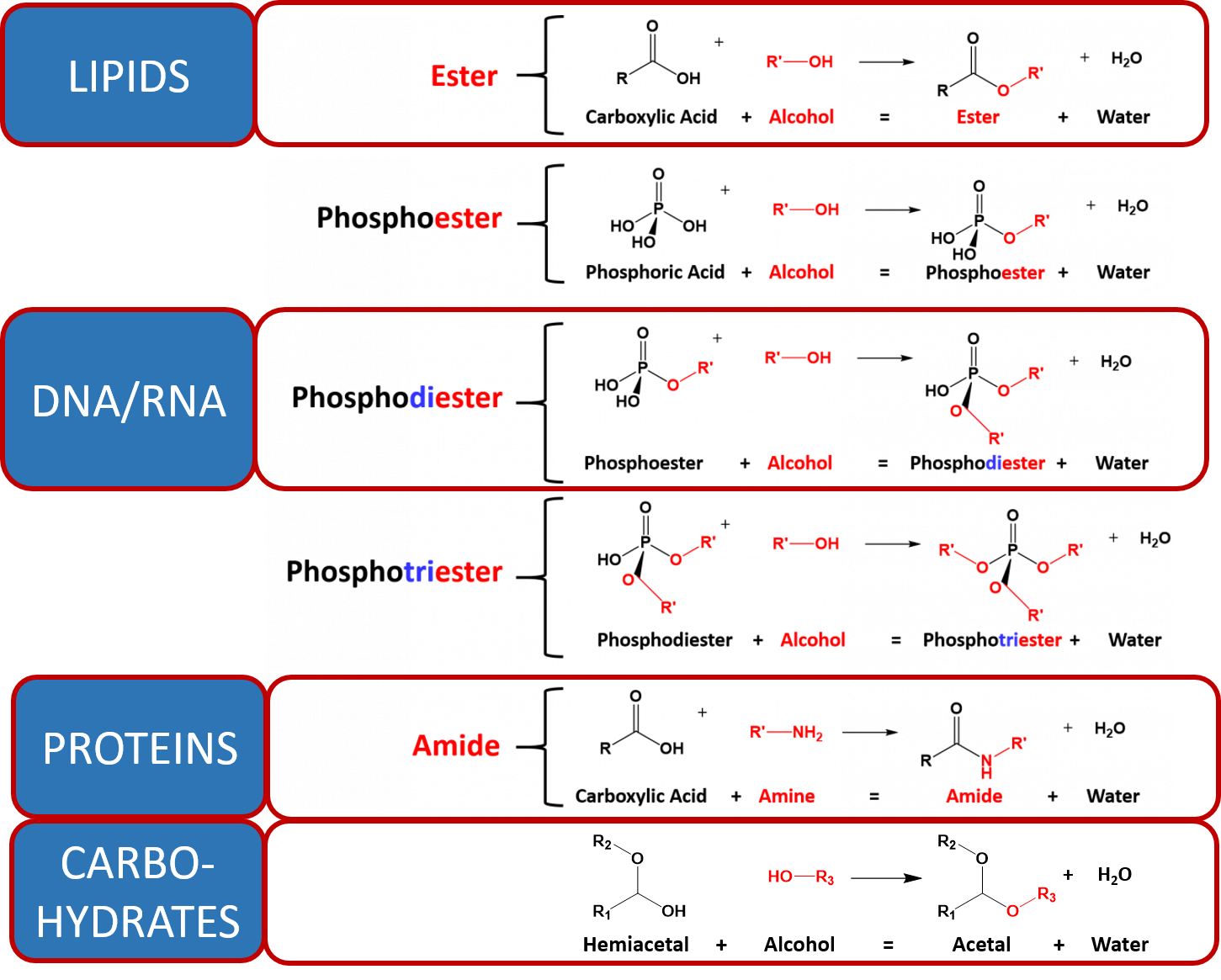
CH103 – Chapter 7: Chemical Reactions in Biological Systems – Chemistry Jul 25, 2022In this reaction, and in most chemical reactions, bonds are broken in the reactants (here, Cr-O and N-H bonds), and new bonds are formed to create the products (here, O-H and N≡N bonds). If the numbers of each type of atom are different on the two sides of a chemical equation, then the equation is unbalanced, and it cannot correctly
Source Image: wou.edu
Download Image
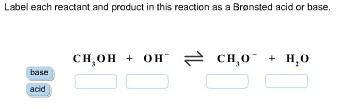
Label each reactant and product in this reaction as a Bronsted acid or base – Home Work Help – Learn CBSE Forum Label each reactant and product in the given chemical reaction. Click the card to flip 👆 A reactant is an element or compound participating in a chemical reaction that undergoes a chemical change, and is shown to the left of the reaction arrow.
Source Image: ask.learncbse.in
Download Image
For the following chemical reaction, label the reactants and | Quizlet All chemical reactions—including a candle burning—involve reactants and products. Reactants are substances that start a chemical reaction. Products are substances that are produced in the reaction. When a candle burns, the reactants are fuel (the candlewick and wax) and oxygen (in the air). The products are carbon dioxide gas and water vapor.

Source Image: quizlet.com
Download Image
CollisionTheorySE Revised For MYP-Gizmos | PDF | Reaction Rate | Chemical Reactions
For the following chemical reaction, label the reactants and | Quizlet Label each reactant and product (as “reactant” or “product“) in the given chemical reaction. CH4 + 2 O2 CO2+ 2 H2O Question 2 of 25
Converting Mass of Reactant to Mass of Product — Examples – Expii Label each reactant and product in this reaction as a Bronsted acid or base – Home Work Help – Learn CBSE Forum Jul 25, 2022In this reaction, and in most chemical reactions, bonds are broken in the reactants (here, Cr-O and N-H bonds), and new bonds are formed to create the products (here, O-H and N≡N bonds). If the numbers of each type of atom are different on the two sides of a chemical equation, then the equation is unbalanced, and it cannot correctly