Jan 18, 2024Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol. If you know how to calculate molar mass, learn about other ways to express the amount of
How many moles are `9.033 xx 10^(24)` atoms of helium (He) ? – YouTube
Aug 10, 2023Wiki User ∙ 13y ago This answer is: More answers Wiki User ∙ 9y ago Copy The molar mass of SiH4 is approximately 32.117 grams per mole. So, 9.30 moles of SiH4 has a mass of 298.69

Source Image: scribd.com
Download Image
Molar mass of SiH4 (Silane) is 32.11726 ± 0.00058 g/mol Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between SiH4 weight and moles Elemental composition of SiH4 Chemical structure Appearance Silane is a colorless, flammable gas with a pungent odor. Sample reactions for SiH4 Related compounds

Source Image: academia.edu
Download Image
Diamond Nanoparticles in Heterogeneous Catalysis | Chemistry of Materials What is the mass of 9.30 moles of SiH4? Instant Video Answer. Instant Text Answer. Step 1/2 1. Find the molar mass of SiH4 (silane). – Si: 28.09 g/mol – H: 4.03 g/mol (4 hydrogen atoms in SiH4) – Molar mass of SiH4 = 28.09 + 4.03(4) = 32.11 g/mol Step 2/2
Source Image: studylib.net
Download Image
What Is The Mass Of 9.30 Moles Of Sih4
What is the mass of 9.30 moles of SiH4? Instant Video Answer. Instant Text Answer. Step 1/2 1. Find the molar mass of SiH4 (silane). – Si: 28.09 g/mol – H: 4.03 g/mol (4 hydrogen atoms in SiH4) – Molar mass of SiH4 = 28.09 + 4.03(4) = 32.11 g/mol Step 2/2 SiH4 Molar Mass SiH4 Oxidation Number. Products. Silicon – Si. Element 14. Si Molar Mass Si Oxidation Number. Dihydrogen – H 2. Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H … 30.5432 kJ/mol-30.5432 kJ: Si (g) 1 mol: 455.6376 kJ/mol: 455.6376 kJ: H 2 (g) 2 mol: 0 kJ/mol:
felder solver
The formula used to calculate mass from moles involves the substance’s molar mass. The formula for calculating Mass (m) using the number of moles (n) and the molar mass (M) of the substance is: Mass (m) = n × M. Where: Mass (m) is the estimated mass of the substance, typically measured in grams (g). Number of Moles (n) is the quantity of Diamond Nanoparticles in Heterogeneous Catalysis | Chemistry of Materials

Source Image: pubs.acs.org
Download Image
PDF) CHAPTER TWO | Bengünur Kırbuğa – Academia.edu The formula used to calculate mass from moles involves the substance’s molar mass. The formula for calculating Mass (m) using the number of moles (n) and the molar mass (M) of the substance is: Mass (m) = n × M. Where: Mass (m) is the estimated mass of the substance, typically measured in grams (g). Number of Moles (n) is the quantity of

Source Image: academia.edu
Download Image
How many moles are `9.033 xx 10^(24)` atoms of helium (He) ? – YouTube Jan 18, 2024Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol. If you know how to calculate molar mass, learn about other ways to express the amount of

Source Image: m.youtube.com
Download Image
Diamond Nanoparticles in Heterogeneous Catalysis | Chemistry of Materials Molar mass of SiH4 (Silane) is 32.11726 ± 0.00058 g/mol Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between SiH4 weight and moles Elemental composition of SiH4 Chemical structure Appearance Silane is a colorless, flammable gas with a pungent odor. Sample reactions for SiH4 Related compounds

Source Image: pubs.acs.org
Download Image
464. What is the mass of 9.30 moles of SiH4? – Brainly.ph Post any question and get expert help quickly. Start learning Answer to Solved What is the mass of 9.30 moles of SiH _ (4) ? | Chegg.com

Source Image: brainly.ph
Download Image
Pressure Dependence of Rate Coefficients of Unimolecular and Chemical Activation Reactions Connected to the Potential Energy Wells of Si2H2Cl4, Si2Cl6, and Si2Cl4 via Rice–Ramsperger–Kassel–Marcus Calculations | The Journal of Physical Chemistry A What is the mass of 9.30 moles of SiH4? Instant Video Answer. Instant Text Answer. Step 1/2 1. Find the molar mass of SiH4 (silane). – Si: 28.09 g/mol – H: 4.03 g/mol (4 hydrogen atoms in SiH4) – Molar mass of SiH4 = 28.09 + 4.03(4) = 32.11 g/mol Step 2/2

Source Image: pubs.acs.org
Download Image
Answered: Tsion Factor Part A What is the mass of… | bartleby SiH4 Molar Mass SiH4 Oxidation Number. Products. Silicon – Si. Element 14. Si Molar Mass Si Oxidation Number. Dihydrogen – H 2. Molecular Hydrogen Hydrogen Molecule Hydrogen Hydrogen Gas Molecular Hydrogen Gas H … 30.5432 kJ/mol-30.5432 kJ: Si (g) 1 mol: 455.6376 kJ/mol: 455.6376 kJ: H 2 (g) 2 mol: 0 kJ/mol:

Source Image: bartleby.com
Download Image
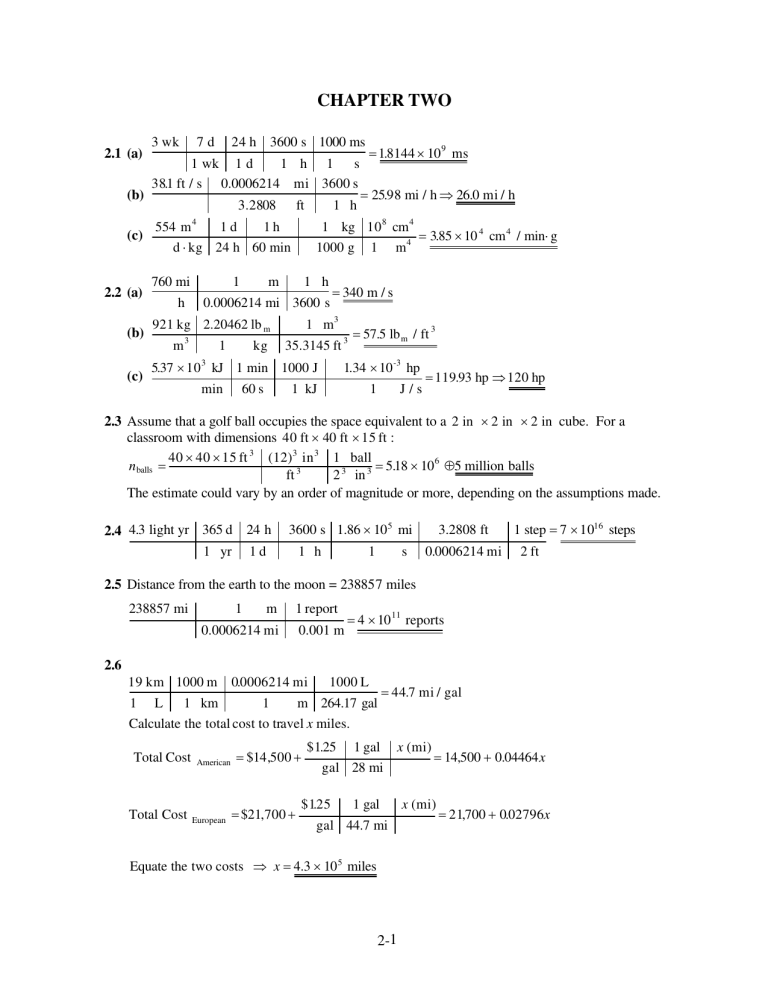
PDF) CHAPTER TWO | Bengünur Kırbuğa – Academia.edu
Answered: Tsion Factor Part A What is the mass of… | bartleby Aug 10, 2023Wiki User ∙ 13y ago This answer is: More answers Wiki User ∙ 9y ago Copy The molar mass of SiH4 is approximately 32.117 grams per mole. So, 9.30 moles of SiH4 has a mass of 298.69
Diamond Nanoparticles in Heterogeneous Catalysis | Chemistry of Materials Pressure Dependence of Rate Coefficients of Unimolecular and Chemical Activation Reactions Connected to the Potential Energy Wells of Si2H2Cl4, Si2Cl6, and Si2Cl4 via Rice–Ramsperger–Kassel–Marcus Calculations | The Journal of Physical Chemistry A Post any question and get expert help quickly. Start learning Answer to Solved What is the mass of 9.30 moles of SiH _ (4) ? | Chegg.com