Find stepby-step Chemistry solutions and your answer to the following textbook question: Complete the Ka2 expression for H2CO3 in an aqueous solution.. … Answered this week. The K a X 2 \ceKa_2 Ka X 2 expression for H X 2 C O X 3 \ceH2CO3 H X 2 CO X 3 in an aqueous solution is: K a X 2 = [H X +]
Dr. Tarek Elbashiti Assoc. Prof. of Biotechnology Environmental conditions of animal cell culture. – ppt download
In each of these examples, the equilibrium system is an aqueous solution, as denoted by the aq annotations on the solute formulas. Since H 2 O(l) is the solvent for these solutions, it is assigned an activity of 1, and thus does not appear explicitly as a term in the \(K_c\) expression, as discussed earlier, even though it may also appear as a
![SOLVED: What is the Ka expression for HCO3 when it acts as a Bronsted-Lowry acid in water: Ka = [HCO3-][H3O+]/[H2CO3]](https://cdn.numerade.com/ask_previews/d22edfc2-cb1b-47b4-af5d-1cb5f609786d.gif)
Source Image: numerade.com
Download Image
Carbonic acid, H X 2 C O X 3 \ceH2CO3 H X 2 CO X 3 , is a diprotic acid which dissociates in two steps and in each step releases 1 H X + \ce1H+ 1 H X + ion. The first equilibrium dissociation reaction is shown below:

Source Image: quora.com
Download Image
Why is an H2CO3 solution acidic? As H2Co3 is in equilibrium with H+ (acid) and HCO3 (base), why doesn’t it become neutral? – Quora When carbon dioxide dissolves in water, it is in chemical balance with carbonic acid: CO2 + H2O <==> H2CO3. The hydration equilibrium constant at 25 ° C is called K h, which in the case of carbonic acid is [H2CO3] / [CO2] ≈ 1.7 × 10 −3 in pure water and ≈ 1.2 × 10-3 in sea water. [6] Therefore, the majority of carbon dioxide is not

Source Image: quora.com
Download Image
Complete The оa2 Expression For H2co3 In An Aqueous Solution.
When carbon dioxide dissolves in water, it is in chemical balance with carbonic acid: CO2 + H2O <==> H2CO3. The hydration equilibrium constant at 25 ° C is called K h, which in the case of carbonic acid is [H2CO3] / [CO2] ≈ 1.7 × 10 −3 in pure water and ≈ 1.2 × 10-3 in sea water. [6] Therefore, the majority of carbon dioxide is not Question: Complete the Ka2 expression for H2CO3 in an aqueous solution. Ka2=4.69×10−11=Complete the Kal expression for H2CO3 in an aqueous solution. Kal=4.45×10−7= Pease help, thanks! Show transcribed image text. There are 2 steps to solve this one. Who are the experts?
Why is an H2CO3 solution acidic? As H2Co3 is in equilibrium with H+ (acid) and HCO3 (base), why doesn’t it become neutral? – Quora
Study with Quizlet and memorize flashcards containing terms like Complete Ka1 expression for H2CO3 in an aqueous solution. H2CO3(aq) + H2O(l) ⇌ HCO−3(aq) + H3O+(aq) HCO−3(aq) + H2O(l) ⇌ CO2−3(aq) + H3O+(aq), Complete Ka2 expression for H2CO3 in an aqueous solution. H2CO3(aq) + H2O(l) ⇌ HCO−3(aq) + H3O+(aq) HCO−3(aq) + H2O(l) ⇌ CO2−3(aq) + H3O+(aq), Is this a SA, WA, SB, or Why is an H2CO3 solution acidic? As H2Co3 is in equilibrium with H+ (acid) and HCO3 (base), why doesn’t it become neutral? – Quora

Source Image: quora.com
Download Image
Solved Complete the Kal expression for H2CO3 in an aqueous | Chegg.com Study with Quizlet and memorize flashcards containing terms like Complete Ka1 expression for H2CO3 in an aqueous solution. H2CO3(aq) + H2O(l) ⇌ HCO−3(aq) + H3O+(aq) HCO−3(aq) + H2O(l) ⇌ CO2−3(aq) + H3O+(aq), Complete Ka2 expression for H2CO3 in an aqueous solution. H2CO3(aq) + H2O(l) ⇌ HCO−3(aq) + H3O+(aq) HCO−3(aq) + H2O(l) ⇌ CO2−3(aq) + H3O+(aq), Is this a SA, WA, SB, or
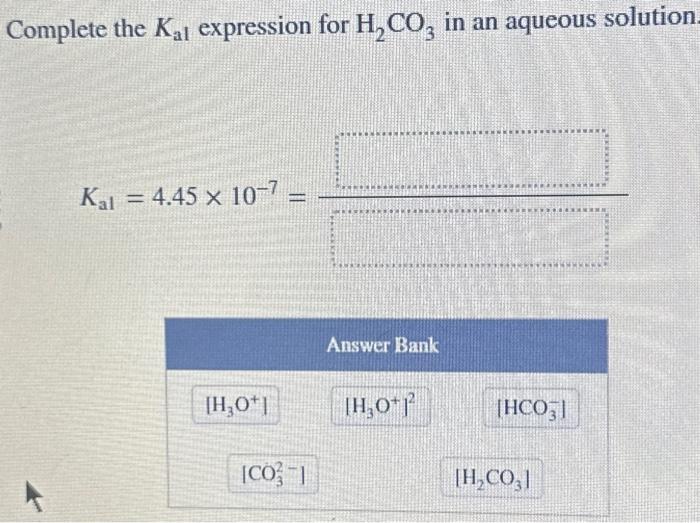
Source Image: chegg.com
Download Image
Dr. Tarek Elbashiti Assoc. Prof. of Biotechnology Environmental conditions of animal cell culture. – ppt download Find stepby-step Chemistry solutions and your answer to the following textbook question: Complete the Ka2 expression for H2CO3 in an aqueous solution.. … Answered this week. The K a X 2 \ceKa_2 Ka X 2 expression for H X 2 C O X 3 \ceH2CO3 H X 2 CO X 3 in an aqueous solution is: K a X 2 = [H X +]

Source Image: slideplayer.com
Download Image
Why is an H2CO3 solution acidic? As H2Co3 is in equilibrium with H+ (acid) and HCO3 (base), why doesn’t it become neutral? – Quora Carbonic acid, H X 2 C O X 3 \ceH2CO3 H X 2 CO X 3 , is a diprotic acid which dissociates in two steps and in each step releases 1 H X + \ce1H+ 1 H X + ion. The first equilibrium dissociation reaction is shown below:

Source Image: quora.com
Download Image
In aqueous solution the ionization constants for carbonic acid a Complete the K_a1 expression for H_2CO_3 in an aqueous solution. Do not include states in the expression. K_a1 = 4.45 \times 10^-7 = \boxed\space Complete the charge balance equation for an aqueous solution of H2CO3 that ionizes to HCO3- and CO32?. Write a net equation for the reaction between aqueous solutions of Na2HPO4 and H2CO3.
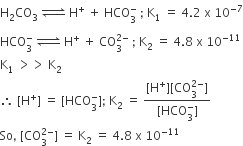
Source Image: zigya.com
Download Image
Solved Question 4 1 pts Consider the following two | Chegg.com When carbon dioxide dissolves in water, it is in chemical balance with carbonic acid: CO2 + H2O <==> H2CO3. The hydration equilibrium constant at 25 ° C is called K h, which in the case of carbonic acid is [H2CO3] / [CO2] ≈ 1.7 × 10 −3 in pure water and ≈ 1.2 × 10-3 in sea water. [6] Therefore, the majority of carbon dioxide is not
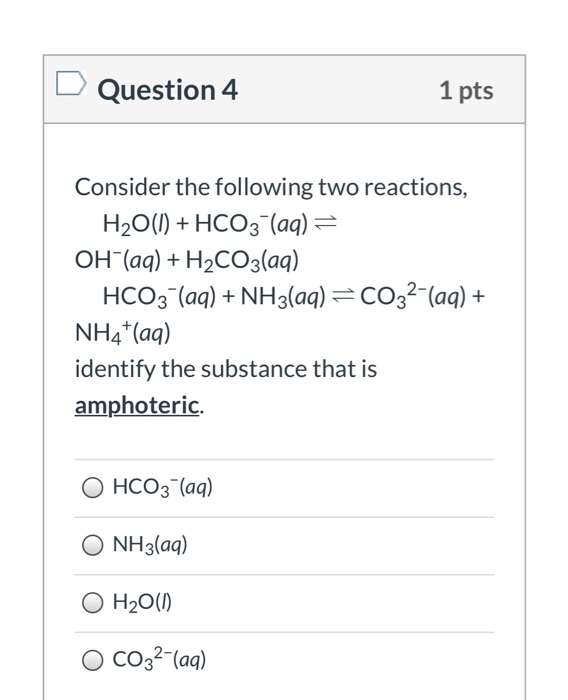
Source Image: chegg.com
Download Image
H2 CO3 (acid)«H+ + HCO3 – (base) – ppt download Question: Complete the Ka2 expression for H2CO3 in an aqueous solution. Ka2=4.69×10−11=Complete the Kal expression for H2CO3 in an aqueous solution. Kal=4.45×10−7= Pease help, thanks! Show transcribed image text. There are 2 steps to solve this one. Who are the experts?

Source Image: slideplayer.com
Download Image
Solved Complete the Kal expression for H2CO3 in an aqueous | Chegg.com
H2 CO3 (acid)«H+ + HCO3 – (base) – ppt download In each of these examples, the equilibrium system is an aqueous solution, as denoted by the aq annotations on the solute formulas. Since H 2 O(l) is the solvent for these solutions, it is assigned an activity of 1, and thus does not appear explicitly as a term in the \(K_c\) expression, as discussed earlier, even though it may also appear as a
Why is an H2CO3 solution acidic? As H2Co3 is in equilibrium with H+ (acid) and HCO3 (base), why doesn’t it become neutral? – Quora Solved Question 4 1 pts Consider the following two | Chegg.com Complete the K_a1 expression for H_2CO_3 in an aqueous solution. Do not include states in the expression. K_a1 = 4.45 \times 10^-7 = \boxed\space Complete the charge balance equation for an aqueous solution of H2CO3 that ionizes to HCO3- and CO32?. Write a net equation for the reaction between aqueous solutions of Na2HPO4 and H2CO3.